| 8.1 |
Discovery of Radioactivity |
The figures involved in the discovery of radioactivity:
|
Figures
|
Discovery
|
|
Willhelm Roentgen
|
Discovered X-rays in 1895
|
|
Henri Becquerel
|
Discovered radioactivity when he discovered uranium salts could darken photo plates in 1896
|
|
Pierre Curie
|
Awarded the Nobel Prize in Physics in 1903, along with henri Becquerel and Marie Curie in radioactivity research
|
|
Marie Curie
|
-
Pioneering
studies of radioactivity
-
Discovered the radioactive elements polonium and radium together with her husband, Pierre Curie
-
She was the first and only woman to win the Nobel prize twice
|
The radioactivity:
-
Radioactivity is a decay process that occurs spontaneously when an unstable nucleus emits radioactive radiation
-
Examples of radioactive materials are carbon-14 (C-14), radon-222 (Rn-222), and uranium-235 (U-235)
-
The radioactivity of a substance is measured in Becquerel units, Bq or in curie units, Ci
The radioactive radiation detection:

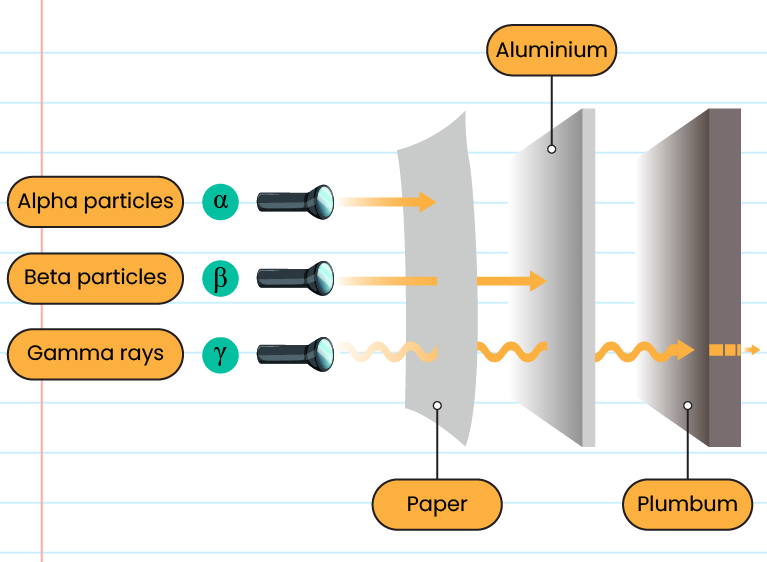
- The three main types of radioactive radiation are alpha (α) radiation, beta radiation (β), and gamma radiation (γ)
The half-life of radioactive decay:
-
Radioactive decay involves the exchange of unstable nucleus to more stable mucus accompanied by radiation of radioactive radiation
-
The half-life of radioactive decay is the time taken for the number of an unstable nucleus in a sample of radioactive material to remain half of its original number
| Radioactive substances |
Half-life |
| Iodin-123 |
13 hours |
| Polonium-210 |
138 days |
| Radium-228 |
5.75 years |
| Amerisium-241 |
432.6 years |
| Carbon-14 |
5730 years |
| Uranium-235 |
703.8 million years |