| 4.2 |
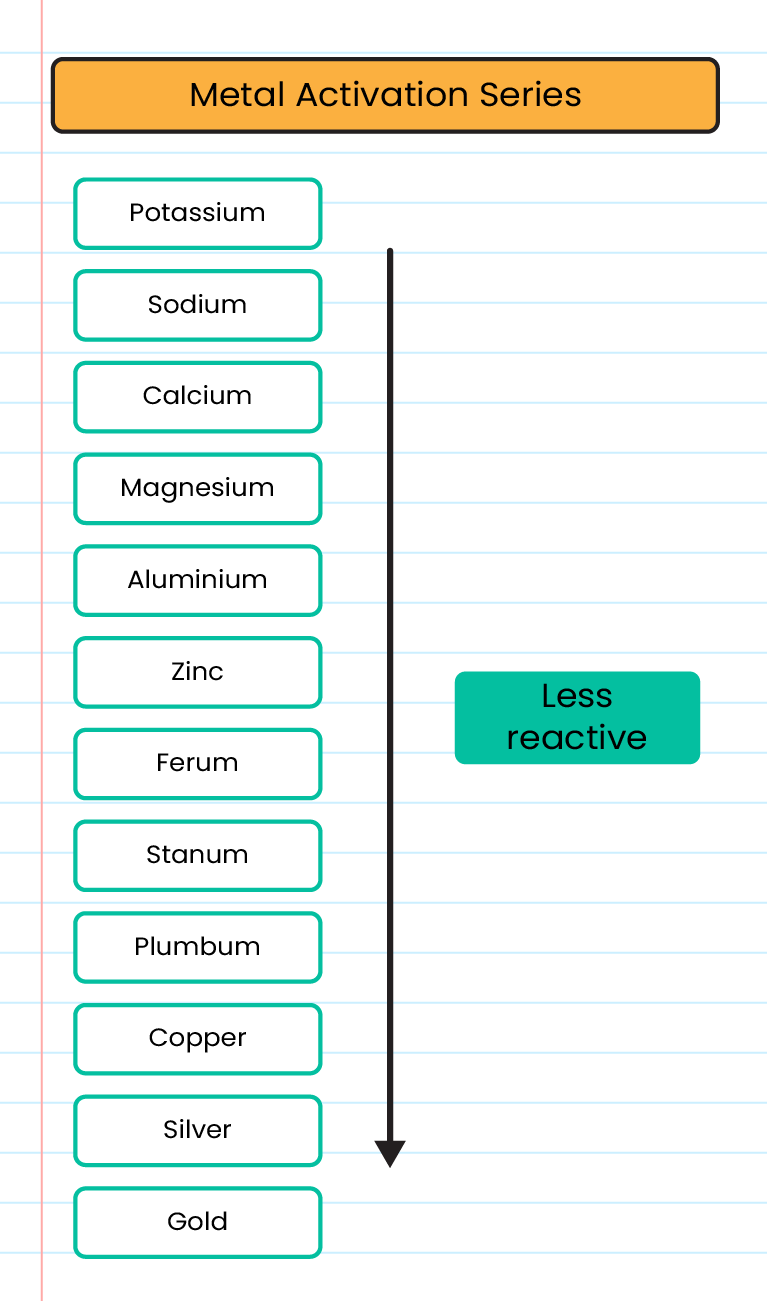
Reactivity Series of Metals |
Metal reactivity series based on reaction with oxygen:
- More reactive metals will remove oxygen from metal oxides
-
Less reactive metals cannot displace more reactive metals than oxide metals
-
A mixture of metal X and metal oxide Y
-
The mixture burns or embers because displacement occurs meaning metal X is more reactive than metal Y
-
No change because displacement does not occur means metal X is less reactive than metal Y
Metal reactivity series based on reaction with hydrogen:
- In addition to the reaction with carbon and oxygen, a series of metal reactivities can also be constructed based on the reaction of a metal with hydrogen
Example question:
A student burns a piece of magnesium tape in a gas jar containing oxygen gas.
This figure shows his observations.
a) Describe the observations shown in the diagram.
b) Write the equation for the reaction that occurs.
Answer:
a) Magnesium burns brightly and very vigorous.
b) \(\begin{aligned} \quad&\text{heat up}\\ \text{Magnesium+oxygen}&\longrightarrow \text{Magnesium oxide} \end{aligned} \)