Chapter 3: Mole Concept, Chemical Formulae and Equations
What you need to learn in this chapter
In this chapter, we will be learning about the concepts of relative atomic mass and relative molecular mass. We will understand how these concepts are based on the carbon-12 scale and learn how to calculate them.
Next, we will dive into the mole concept, defining what a mole is and how it relates to the Avogadro constant, number of particles, and number of moles.
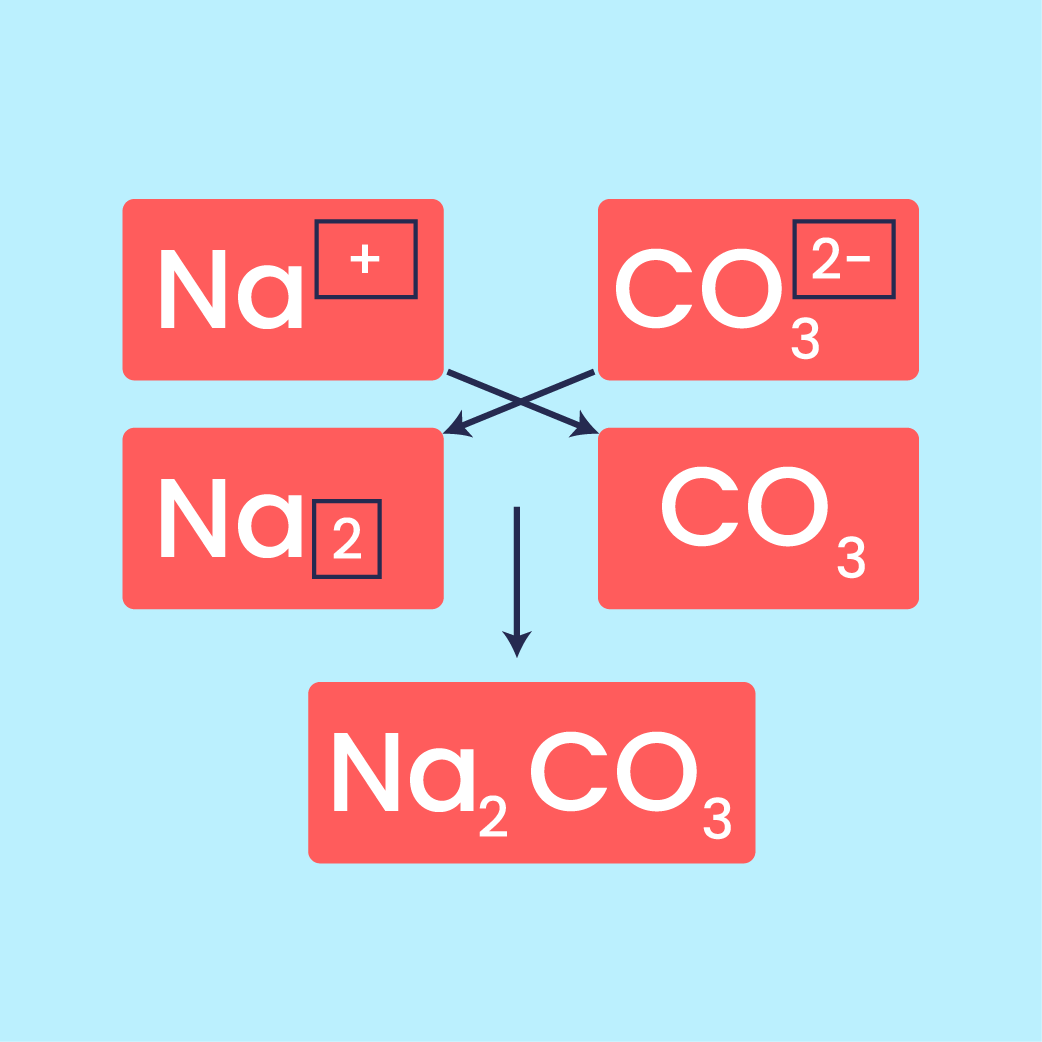
Moving on, we will explore chemical formulae, including the meaning of chemical formula, empirical formula, and molecular formula. Through activities, we will determine the empirical formula of magnesium oxide (MgO).
Lastly, we will learn how to write balanced chemical equations and interpret them qualitatively and quantitatively.
Topics in this chapter
Live Tuition Recordings


Evaluate your academic performance through detailed report
Learn more »
