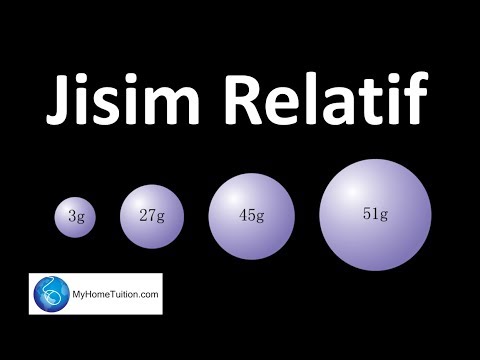
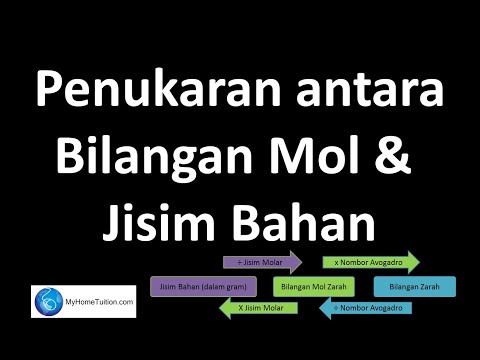
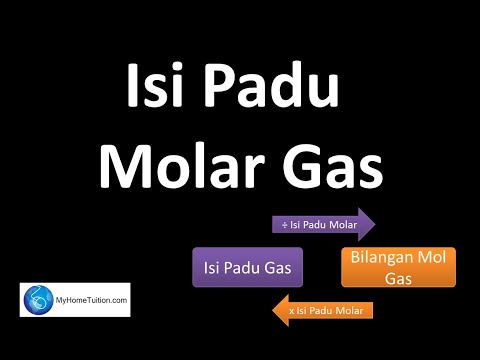
Chapter 3 : Konsep Mol, Formula dan Persamaan Kimia
What will you learn in this chapter
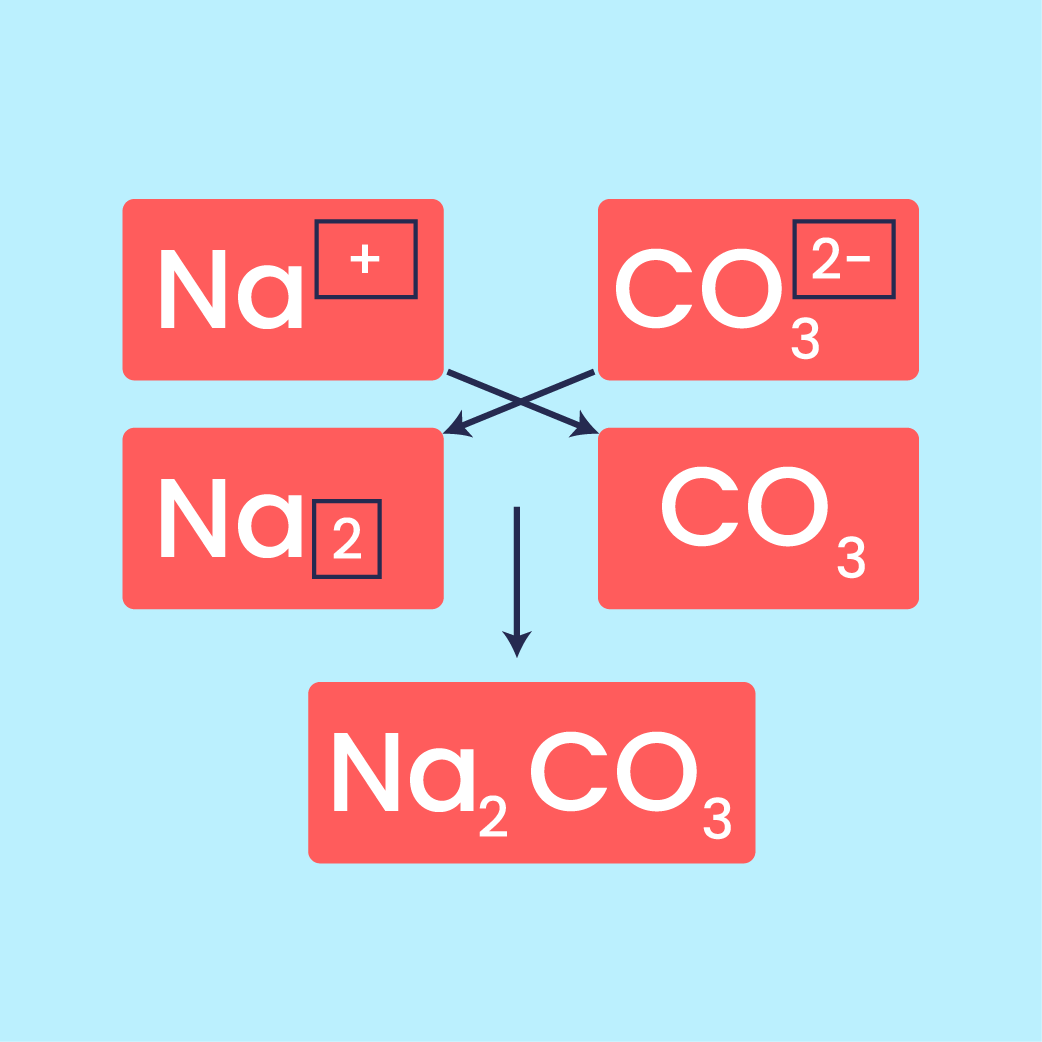
Seterusnya, kita akan menyelami konsep mol, mentakrifkan apa itu mol dan bagaimana ia berkaitan dengan pemalar Avogadro, bilangan zarah dan bilangan mol.
Seterusnya, kita akan meneroka formula kimia, termasuk maksud formula kimia, formula empirikal dan formula molekul. Melalui aktiviti, kita akan menentukan formula empirikal magnesium oksida (MgO).
Akhir sekali, kita akan belajar cara menulis persamaan kimia seimbang dan mentafsirnya secara kualitatif dan kuantitatif.
Topics in this chapter
Live Tuition Recordings

Determining the empirical formula of magnesium oxide (MgO) through activities
Tutor: Sir Aiman
Wednesday
24 Apr 2024
09:00 pm
Experiments
Videos
Practices for this chapter
-
Flashcard
- Chemical Formulae (2)
- Formula Kimia (1)
- Formula Kimia (3)
- Formula Kimia (4)
- Formula Kimia (5)
- Formula Kimia (6)
- Jisim Atom Relatif dan Jisim Molekul Relatif (1)
- Jisim Atom Relatif dan Jisim Molekul Relatif (2)
- Jisim Atom Relatif dan Jisim Molekul Relatif (3)
- Jisim Atom Relatif dan Jisim Molekul Relatif (4)
- Jisim Atom Relatif dan Jisim Molekul Relatif (5)
- Jisim Atom Relatif dan Jisim Molekul Relatif (6)
- Konsep Mol (1)
- Konsep Mol (2)
- Konsep Mol (3)
- Konsep Mol (4)
- Konsep Mol (5)
- Konsep Mol (6)
- Persamaan Kimia (1)
- Persamaan Kimia (2)
- Persamaan Kimia (3)
- Persamaan Kimia (4)
-
Topical Test
- 3.1 Jisim Atom Relatif dan Jisim Molekul Relatif - Set 1
- 3.1 Jisim Atom Relatif dan Jisim Molekul Relatif - Set 2
- 3.2 Konsep Mol - Set 1
- 3.2 Konsep Mol - Set 2
- 3.2 Konsep Mol - Set 3
- 3.2 Konsep Mol - Set 4
- 3.2 Konsep Mol - Set 5
- 3.3 Formula Kimia - Set 1
- 3.1 Formula Kimia - Set 2
- 3.1 Formula Kimia - Set 3
- 3.1 Formula Kimia - Set 4
- 3.4 Persamaan Kimia - Set 1
- 3.4 Persamaan Kimia - Set 2
- 3.4 Persamaan Kimia - Set 3