| Energy Level Diagram |
- During a chemical reaction, heat is absorbed or released.
- This heat is called heat of reaction, and is given the symbol \(\Delta \text{H}\).
- The unit for heat of reaction is \(\text{kJ mol}^{−1}\).
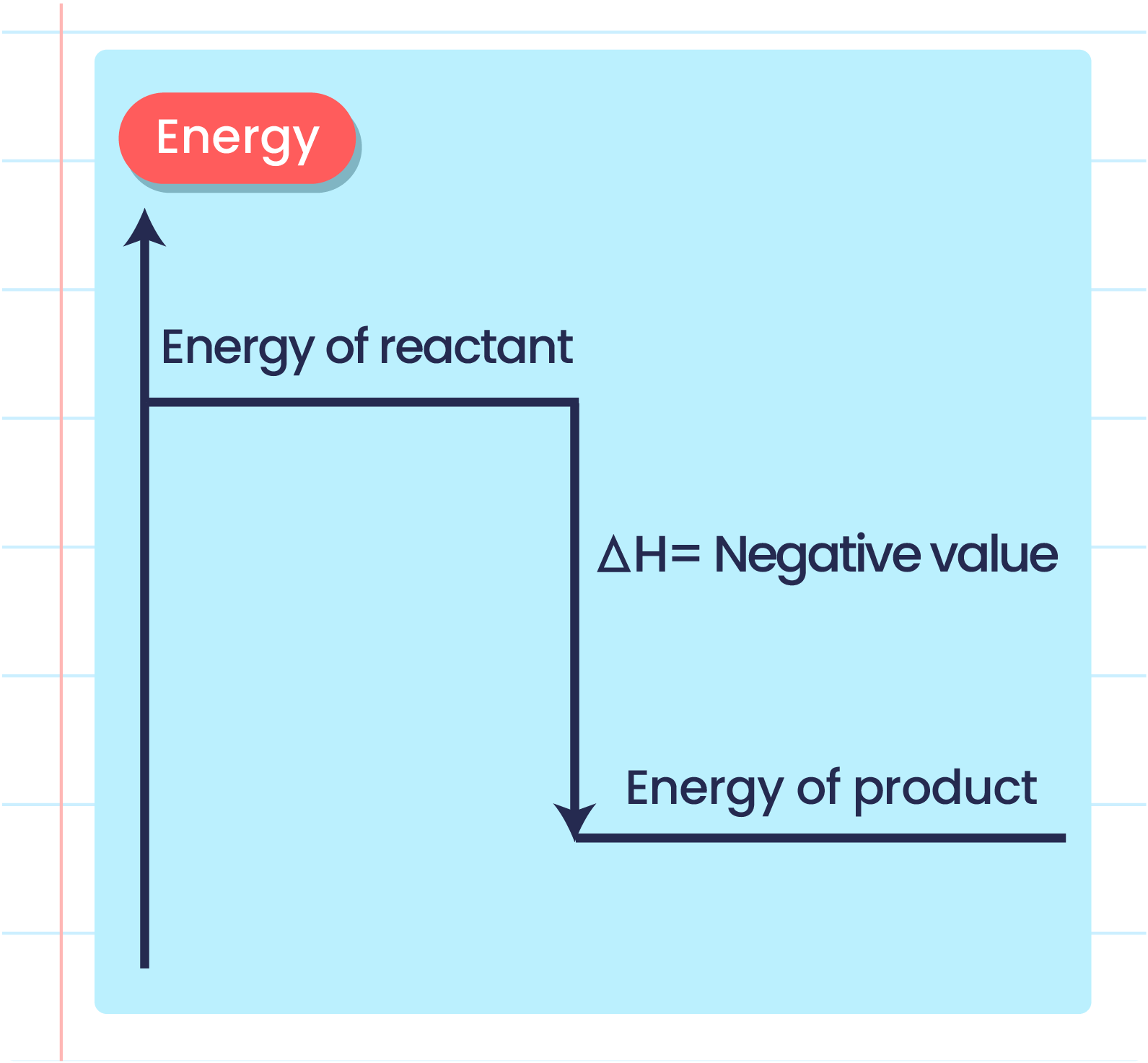
- In a chemical reaction, when heat is released to the surroundings, \(\Delta \text{H}\) is given a negative sign.
- When heat is absorbed from the surroundings, \(\Delta \text{H}\) is given a positive sign.
- The energy change in a chemical reaction can be represented using an energy level diagram.
- The energy level diagram shows the difference in the heat energy content between the reactants and the products.
- The definition of heat of reaction is as stated below:
| Definition of Heat of Reaction, \(\Delta H\) |
|
The heat change of one mole of reactant that reacts or one mole of product that is formed
\(\Delta \text{H}= \text{H}_{\text{product}}-\text{H}_{\text{product}}\)
|
- Example of energy level diagram of the exothermic reaction:
| Energy Level Diagram of the Exothermic Reaction |
 |
|
|