| |
|
|
| |
| Definition of Rusting of Iron |
| A metal corrosion that occurs to iron. |
|
| |
| Process of Rusting of Iron |
- When iron metalrusts, a layer of reddish-brown iron oxide isformed on the iron surface that is easily cracked and permeable.
- Thus,rusting occurs continuously and damage the structure of the iron.
|
|
| |
| Conditions for Rusting of An Iron |
|
|
|
| |
 |
| |
| Redox Reaction of Rusting |
| Step |
Explaination |
|
Oxidation
|
- Iron atom releases two electrons to form iron(II) ion, \(Fe^{2+}\).
- \(Fe(s)\rightarrow Fe^{2+}(aq)+2e^-\\\)
- The released electrons would flow through the iron to the surface.
- Iron is an electrical conductor, thus the electrons are able to flow through the iron.
- There is oxygen gas at the surface.
|
|
Reduction
|
- The released electrons from the oxidation process are received by oxygen gas.
- Since oxygen gas received electrons, oxygen is reduced to form hydroxide ion, \(OH^-\).
- \(O_2(g)+2H_2O(l) +4e^-\rightarrow 4OH^-(aq)\)
|
|
Formation of a chemical cell
|
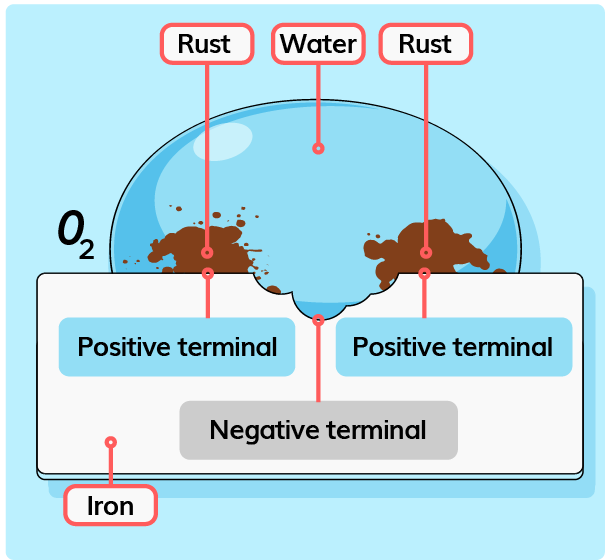
- The part of the system that undergoes oxidation is the negative terminal of the chemical cell.
- The part of the system that undergoes reduction is the positive terminal of the chemical cell.
- \(2\times [Fe(s)\rightarrow Fe^{2+}(aq)+2e^-]\\ O_2(g)+2H_2O(l) +4e^-\rightarrow 4OH^-(aq)\\ \text{Equals to}\\2Fe(s)+O_2(g)+2H_2O(l)\rightarrow 2Fe^{2+}(aq)+4OH^-(aq) \)
|
|
Formation of rust
|
- The iron(II) ions, \(Fe^{2+}\) flow from the negative terminal to the positive terminal.
- At the positive terminal, the iron(II) ions, \(Fe^{2+}\) reacts with the hydroxide ion, \(OH^-\) to form iron(II) hydroxide, \(Fe(OH)_2\).
- \(Fe(aq)+2OH^-(aq)\rightarrow Fe(OH)_2(s)\\\)
- The iron(II) hydroxide is oxidised to form iron(III) hydroxide, \(Fe(OH)_3\).
- \(4Fe(OH)_2(s)+O_2(g)+2H_2O(l)\rightarrow 4Fe(OH)_3(s)\\\)
- The iron(III) hydroxide, \(Fe(OH)_3\). then forms rust.
- Rust is hydrated iron(III) hydroxide, \(Fe_2O_3.3H_2O\).
- \( 4Fe(OH)_3(s)\rightarrow Fe_2O_3.3H_2O(s)\\ \phantom{ 4Fe(OH)_3(s)\rightarrow}Rust\)
|
|
| |
| Formation of Rust |
 |
|
| |
| Definisi of Metal Corrosion |
- Oxidation of metal through the action of air or oxygen gas, water and/or electrolyte.
- The oxidised metal would release electron(s) to form an ion.
|
|
| |
| Metal Corrosion |
- In general, the more electropositive the metal is, the easier it is for the metal to corrode.
- For example, corrosion of iron, Fe is faster than copper, Cu.
|
|
| |
| Prevention of Metal Corrosion |
| Using A More Electropositive Metal |
- Metal corrosion can be prevented by touching a more electropositive metal with the metal being protected.
- The more electropositive metal releases electrons easier.
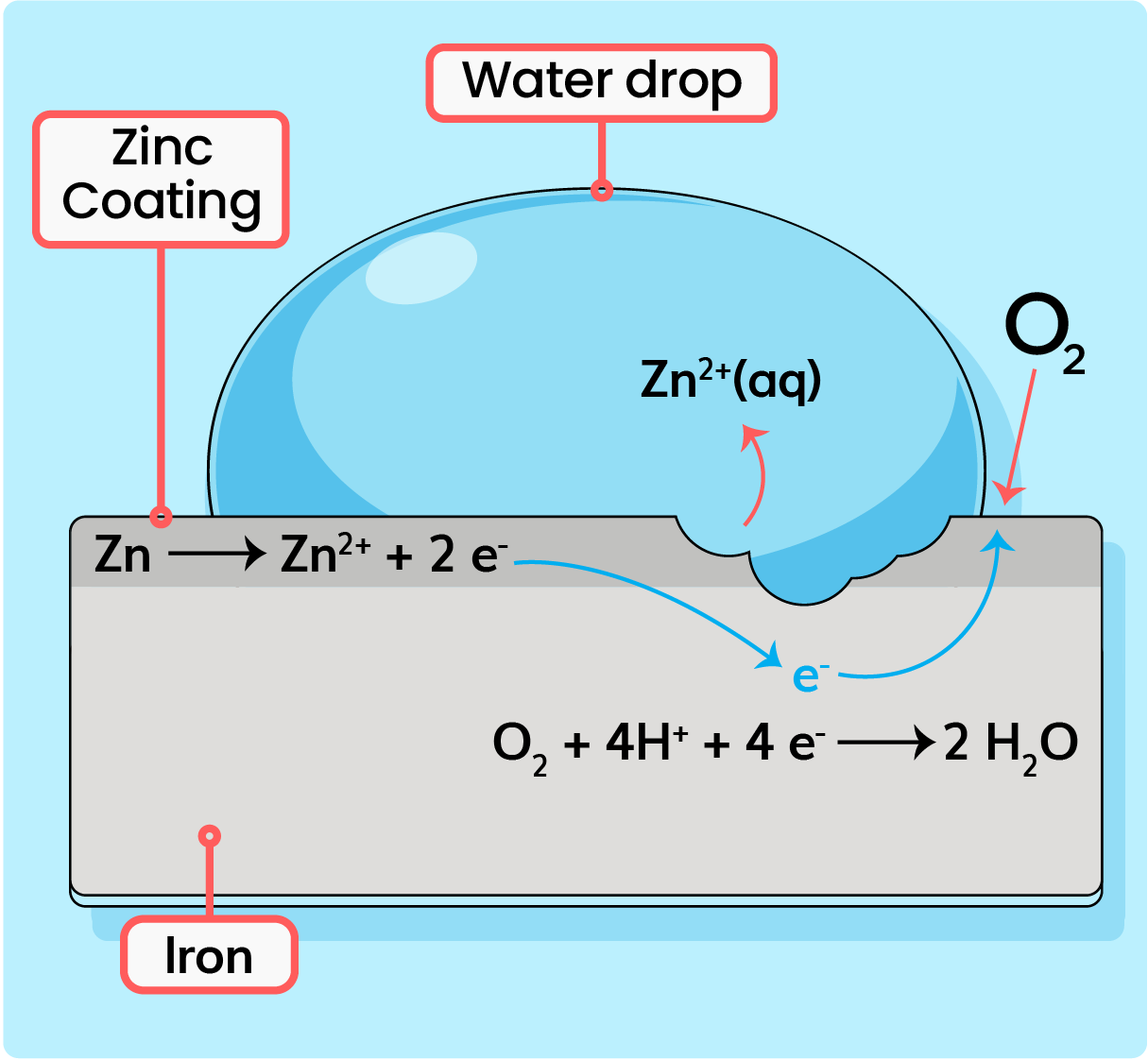
- For example; a layer of zinc protecting an iron, but there is a scratch on the layer of zinc.
- Zinc is more electropositive than iron.
- Zinc is oxidised by releasing electrons.
- Electrons flow to the surface of iron where there are water and oxygen.
- Instead of iron being oxidised, the zinc layer acts as a sacrificial layer of protection as shown below:
| Zinc Layer as A Sacrificial Layer of Protection |
 |
|
| Using A Less Electropositive Metal |
- A layer of less electropositive metal (e.g. tin) protecting iron can still prevent corrosion of iron.
- However, if the protective layer is scratched, the corrosion of iron is enhanced instead of prevented.
- The more electropositive metal releases electrons easier.
- For example; a layer of tin protecting an iron, but there is a scratch on the layer of tin.
- Iron is more electropositive than tin.
- Iron is oxidised by releasing electrons.
- Electrons flow to the surface of iron where there are water and oxygen.
- Instead of tin being oxidised, the iron is oxidised at a faster rate because iron is more electropositive than tin.
|
|
| |